While vaccines are struggling to gain acceptance after a long wait, a laboratory based in Nantes claims to have developed a drug against Covid-19. It is possible that this treatment will be the subject of large-scale production. However, it is incumbent on us to wait for the green light from the French Medicines Agency as well as pre-orders from the French State.
For more than a year, the Covid-19 pandemic has continued to claim victims and disrupt the daily lives of the living . Since January 2021, vaccines are finally available but several details mean that it is not yet a solution to the health crisis, at least in France. Indeed, vaccinations are done by age categories and the delivery of doses is subject to hazards. Let’s also mention the current controversy around the AstraZeneca vaccine. Let us also mention the fact that active people – that is to say workers – are still not concerned by vaccination.
Faced with this situation, there may be hope. In a newsletter published in March 2021, the Xenothera laboratory based in Nantes mentioned its drug against coronavirus SARS-CoV-2. Odile Duvaux, president of this establishment, commented on the results of the first phase of the clinical trial. According to the person concerned, the drug in question is effective and claims to receive a temporary authorization for use from the National Agency for the Safety of Medicines and Health Products (ANSM).
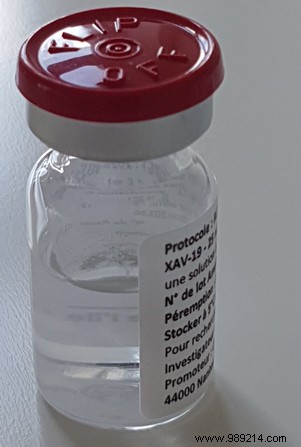
CHU7 Nantes led the first phase of the clinical trial of this drug – Xav-19. Tested on 398 patients in 35 hospitals , the treatment presents polyclonal antibodies. It is a cocktail of antibodies whose mission is to attack the coronavirus. Nevertheless, the results of the first phase have not been published and Odile Duvaux explained why in an article published by France 3 Régions on March 25, 2021.
“This is a precautionary principle that applies almost everywhere in the world. Yet, in the face of this epidemic, England and most other countries publish the results of their studies within a month of the conclusion of the trials. »
While Xenothera waits for ANSM authorization, it is also waiting for pre-orders from the French State and possibly other countries. In the event that these conditions are met, the Xav-19 could well be the subject of large-scale production. Obviously, this treatment does not prevent Covid-19 and is not intended to replace vaccines. On the other hand, treat the disease in case of infection is a very interesting promise with the aim of avoiding more deaths, while waiting for vaccination campaigns to reach their goal.