As France wakes up in near-total lockdown on Tuesday, researchers continue to work hard to come up with a vaccine for the Covid coronavirus -19. This Monday, an American team even started a first clinical trial.
To develop a vaccine, researchers usually rely on the viruses themselves. The idea, roughly speaking, is to "damage" them enough to ensure very low replication (or even zero). Through this method, we can expose, and therefore accustom, our immune system to the proteins produced by the pathogen. Later, our natural defenses will then be able to recognize this enemy if it presents itself, and to trigger an immune response.
The downside of this method is that simply isolating and manipulating the pathogen, then culturing it on a large scale, is very time consuming. .
Some viruses may only have a limited number of proteins on the surface to which the immune system is able to respond. In these cases, it is then possible for researchers to focus only on the genes of these proteins , producing large amounts of protein itself. Nevertheless, while the technique saves some time, it still involves challenges with large-scale production.
But there is another approach, which has been known for a few years, which allows you to simplify the process even more. Why ? Because it allows the development of a treatment as soon as the virus genome is sequenced . This was done by the Institut Pasteur in Wuhan (China) several weeks ago.
Proteins are produced by translating the sequence of a molecule called messenger RNA, usually transcribed from a gene on the body's chromosome. What a virus does when it enters our body is that it orders such and such a cell to manufacture its proteins by creating messenger RNAs from its own genome.
This new approach, which is discussed here, therefore aims to make many copies of the messenger RNAs that said virus uses, and then package them in a vaccine. After injection into the body, these copies enter our cells and encourage them to manufacture viral proteins.
The advantage is that without the rest of the virus, these proteins remain harmless. And in the event of a new attack by the pathogen, our natural defenses will be able to react insofar as they have already been exposed to these proteins.
However, not everything is so simple. You should know that our immune system generally interprets RNA molecules coming from outside cells as threats. This is why he ensures that these are destroyed. To anticipate losses, you must therefore produce a lot of these molecules .
And then, you also have to know that RNA molecules are very charged, which makes their passage through the fatty membranes of a cell very complicated. The idea is then to encapsulate them in small pockets of fat. These will then fuse with the cell membranes, more easily transferring messenger RNAs inside, where they can begin the production of viral proteins.
The Moderna Company, which focused on this approach, succeeded in overcoming all these challenges to finally develop an experimental vaccine named mRNA-1273 . A first phase I trial has just started, led by the Kaiser Permanente Washington Research Institute in Seattle (USA), with the aim of identifying tolerated doses without side effects. The idea will be to offer an injection to 45 healthy adults aged 18-55 .
The first two of these patient volunteers are a man, named Neal Browning, and a woman named Jennifer Haller, COO of a small tech company. “We all feel so helpless. This is an incredible opportunity for me to be able to do something », she said.
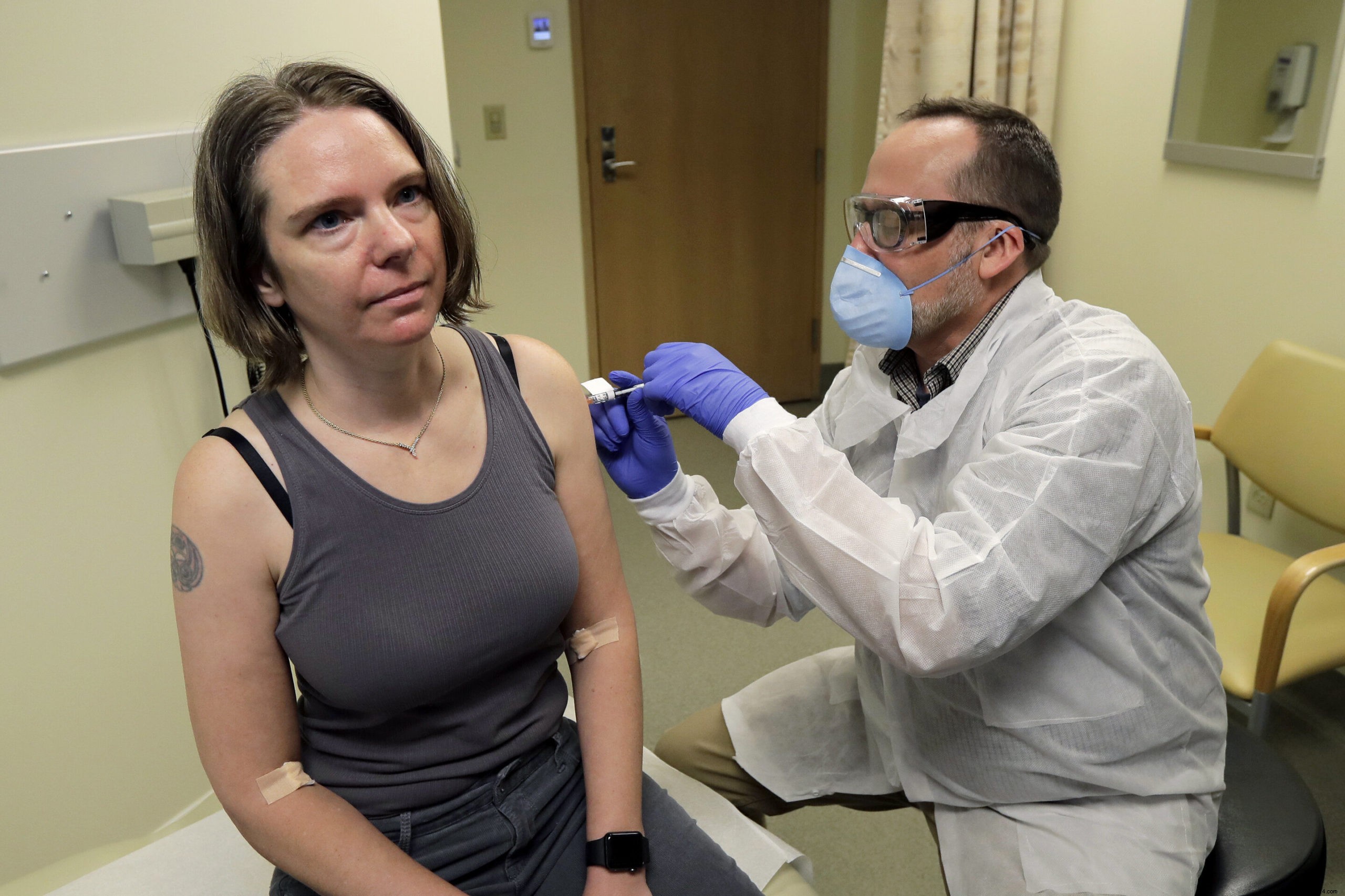
Calendar side, if no problem found during phase I, phase II can then begin. In this case, a larger number of patients will be treated to assess the true immune response of the vaccine. Moderna is already preparing the doses, and is ensuring that it has the necessary facilities to significantly increase production if necessary.
If successful, then we could have a vaccine available for widespread use within 12-18 months.
Source
Related Articles: